FDA advisers reject Biden's plan to offer Pfizer boosters for all, embraces 3rd shots for older, at-risk Americans only - ABC News

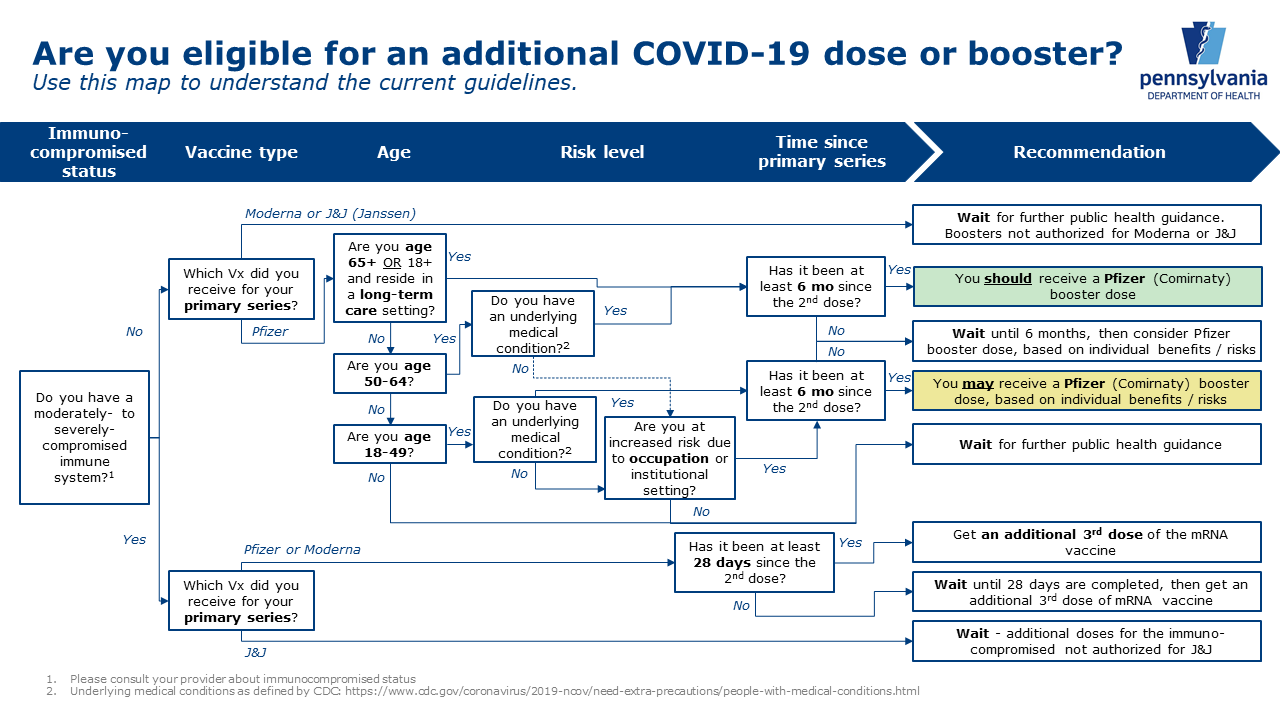
U.S. FDA on X: "Today, we amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to allow for use of a single booster dose, to be administered at least 6

FDA authorizes new guidance on Pfizer vaccines and boosters for children and adults : Oregon Health News Blog
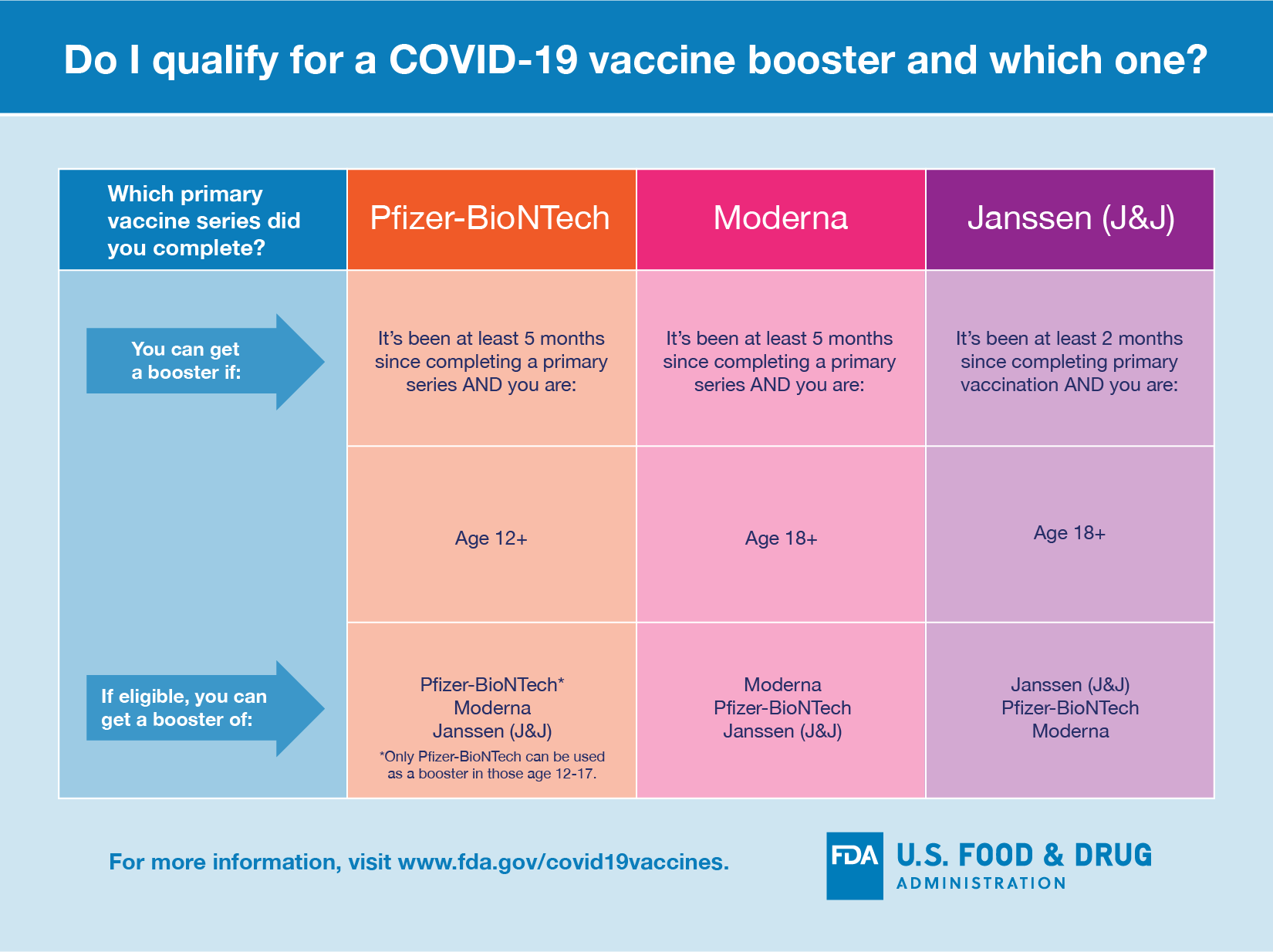
Coronavirus (COVID-19) Update: FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months | FDA
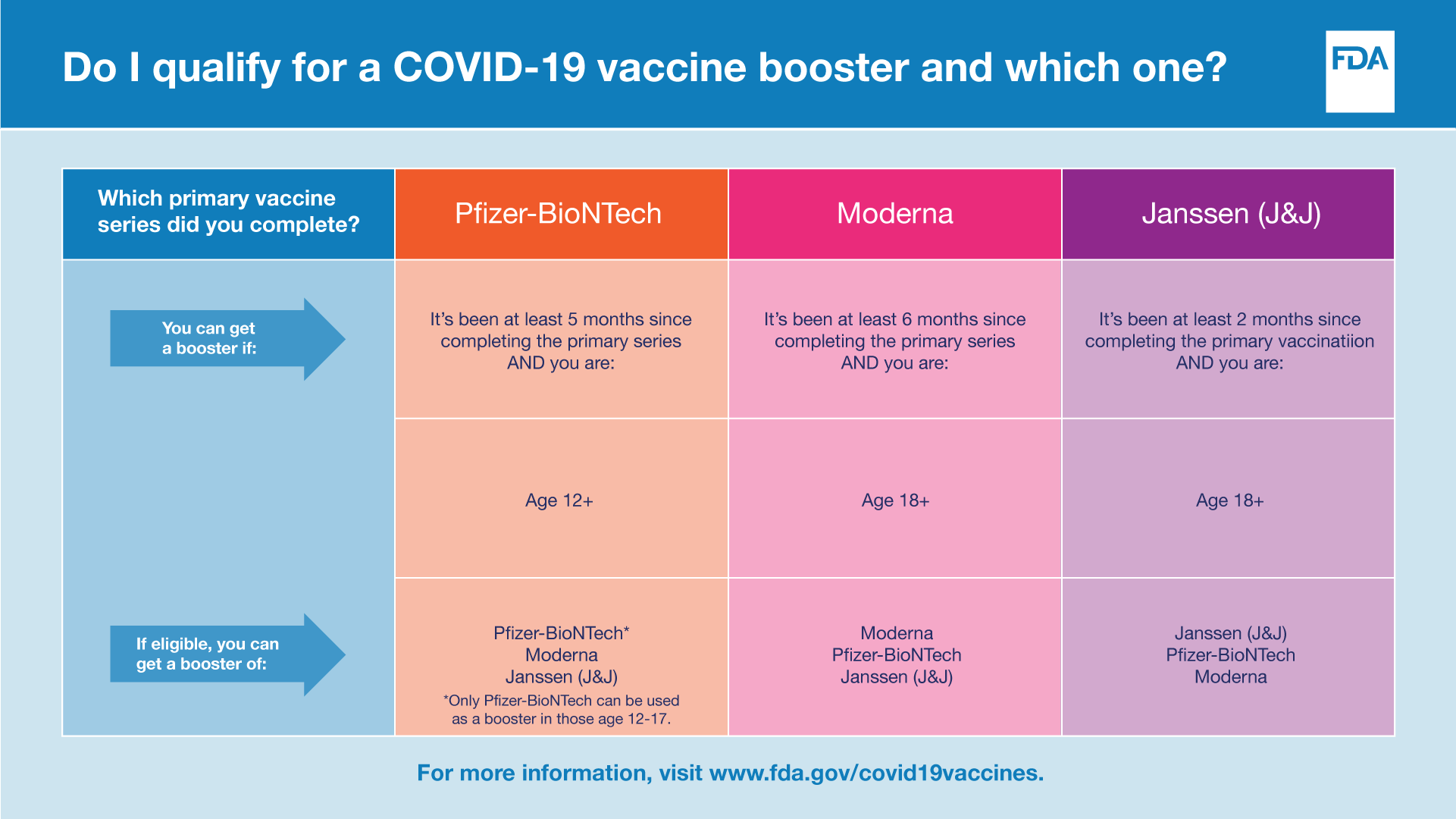
Coronavirus (COVID-19) Update: FDA Takes Multiple Actions to Expand Use of Pfizer-BioNTech COVID-19 Vaccine | FDA

Tense decision-making as CDC joins FDA in recommending Pfizer booster shot for 65 & up, people at high risk and those with occupational exposure to COVID-19